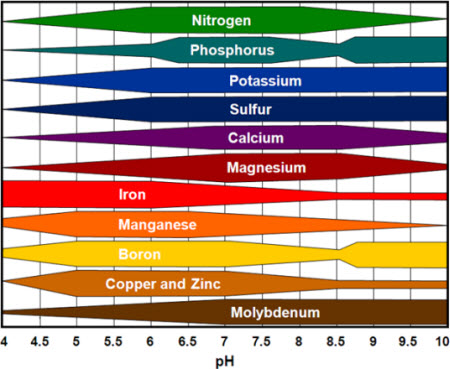
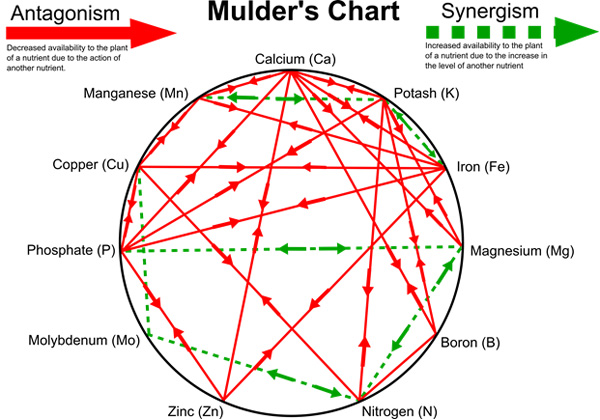
Each essential element has at least one specifically defined role in plant growth so that plants fail to grow and reproduce normally in its absence. Most of the essential elements have several functions in the plant.
Carbon, from carbon dioxide (CO2) in the atmosphere, is assimilated by plants in the photosynthetic process. It is a component of organic compounds such as sugars, proteins, and organic acids. These compounds are used in structural components, enzymatic reactions, and genetic material.
Oxygen, derived from CO2, also is a part of organic compounds such as simple sugars. Atmospheric oxygen is necessary for all oxygen-requiring reactions in plants including nutrient uptake by roots.
Hydrogen derived from water (H2O) is incorporated into organic compounds in the photosynthetic process. Hydrogen is involved in electrochemical reactions and maintain electrical charge balances across all membranes.